GMP Certification in the Philippines: Requirements, Benefits & Process

Introduction
These days, it’s not just about making safe, high-quality products that are compliant—it’s a necessity for success. For Philippine manufacturers, GMP certification provides a compelling method to demonstrate you’re meeting these international requirements. Whether your business is in food, pharmaceuticals, cosmetics, or supplements, becoming certified in Good Manufacturing Practice (GMP) can lead to access to new markets, customer confidence, and regulatory acceptance.
This article deconstructs what exactly GMP certification is, how it benefits, and how to get certified in the Philippines.
What is GMP Certification?
GMP for Good Manufacturing Practice, a system of quality that regulates the manufacturing and testing of products to make them safe and consistent.
GMP regulations address all stages of the production process—right from handling raw materials to sanitation, paperwork, and end-product packaging. The aim is straightforward: avoid contamination, minimize mistakes, and make products consistently with high quality.
Internationally, GMP is known and frequently mandated by regulatory bodies such as the FDA, WHO, and EU authorities. Locally in the Philippines, GMP standards are consistent with those international standards, allowing local business to comfortably export globally.
Request A Free Quote
Why GMP Certification Matters in the Philippines
GMP certification in the Philippines isn’t merely a quality seal—it’s a business advantage.
- Required in regulated industries: The Philippine FDA (previously BFAD) mandates GMP compliance for pharmaceuticals, food, and cosmetics.
- Provider of assurance to retailers and consumers: Certification lends credibility to your product label.
- Export requirement: Most countries will not accept imported products unless they are certified GMP.
- Necessary for government contracts and tenders: Many public and private institutions require GMP-certified suppliers for procurement.
Being GMP certified distinguishes your business from the competition and demonstrates your dedication to quality and safety.
You can also refer to the GMP Wikipedia page for a general overview of its global application and historical development.
Advantages of GMP Certification
Investing in GMP certification can yield several long-term advantages:
- Enhanced product safety and reliability
- Reduced chances of recalls, complaints, and non-compliance
- Greater regulator and customer confidence
- More robust internal quality systems
- International export and high-value contract eligibility
It also supports ASEAN harmonization initiatives for Filipino firms, thus facilitating regional trade.
GMP Certification Requirements in the Philippines
For certification, your firm should satisfy certain GMP standards across a number of key areas:
1. Facility and Equipment
- Well-ventilated, clean, and well-organized manufacturing space
- Preventative hygienic design to eliminate cross-contamination
- Adequate lighting, waste disposal, and pest control systems
2. Personnel Hygiene and Training
- All personnel are required to adhere to hygiene practices
- Appropriate clothing, protective clothing, and health surveillance
- Regular training on GMP procedures and safety measures
3. Documentation and Records
- Comprehensive Standard Operating Procedures (SOPs)
- Batch production and quality control records
- Employee training records and maintenance records
4. Product Handling and Storage
- Suitable storage conditions (temperature, humidity)
- Traceability and proper labeling
- Complaint handling and recall procedures
How to Get GMP Certified in the Philippines
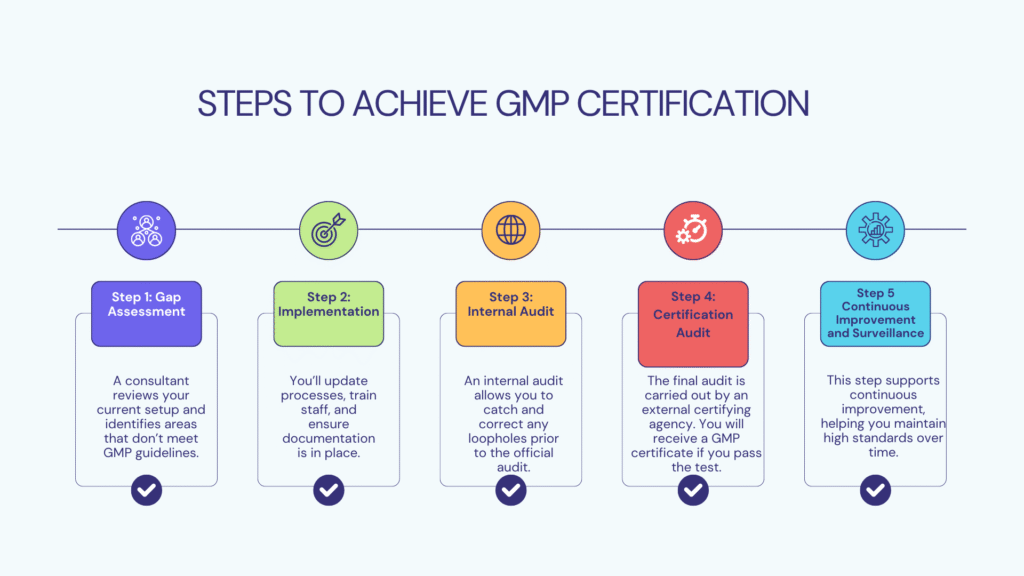
Who Should Obtain GMP Certification in the Philippines?
GMP is necessary for companies in the following sectors:
- Food and beverages processing
- Pharmaceutical production
- Cosmetics and personal care products
- Nutritional supplements
- Herbal products and traditional medicine
- Medical device manufacturing
Even small or medium-scale enterprises (SMEs) stand to gain, particularly when selling to wholesalers or exporting.
Challenges Common to All and How to Fix Them
Most companies experience challenges while working towards certification:
- Obsolescence of documentation or SOPs not in place
- Insufficient staff training
- Poor facility planning or cleanliness
- Less than consistent quality control checks
How to fix them:
- Recruit a professional consultant
- Conduct mock audits
- Engage leadership in quality activities
- Refresh your systems on a regular basis
How Long Does It Take to Get Certified?
The average GMP certification cycle in the Philippines is 3 to 6 months, depending on:
- Your facility’s current situation
- Your documentation level
- The speed at which your team becomes familiar with GMP practices
- Readiness for audit
What Does GMP Certification Cost in the Philippines?
Fees differ based on company size and complexity. Plan for:
- Pre-audit and consultancy charges
- Setup of training and documentation
- Audit and certification charges
While the initial investment might be high, the long-term ROI is substantial, particularly if you’re venturing into regulated or foreign markets.
How Maxicert Can Assist
We at Maxicert assist Philippine businesses throughout the whole GMP certification process:
- Gap analysis and consultation
- Document preparation
- Employee training and awareness
- Mock audits to confirm preparedness
- Accredited certification with trusted partners
We assist companies in food, pharma, and personal care sectors achieve GMP requirements and succeed in competitive markets.
Conclusion
Getting GMP certification in the Philippines is not only a matter of compliance with the rules, but also a forward-thinking decision that enhances your operations, builds consumer confidence, and leads to high-value markets. If you’re in the food, pharma, cosmetics, or health products business, applying GMP is an effective move towards sustaining business growth.
At Maxicert, we make the road to compliance easy with expert advice, customized documentation, employee training, and audit preparedness assistance—so you can concentrate on what matters most: producing safe, high-quality goods.
Ready to be GMP certified in the Philippines?
Get a Free GMP Certification Consultation Now and embark on the journey to international compliance and credibility.

Get In Touch

Get In Touch

Get In Touch
Need A Free Estimate?
Get a free consultation and Checklist to get certified for ISO , HALAL, CE Mark Certification.
FAQ
Is GMP certification mandatory in the Philippines?
Yes, for certain industries like food, pharma, and cosmetics. It’s typically required under FDA regulations.
Who offers GMP certification in the Philippines?
Accredited third-party organizations, usually licensed by FDA or overseas regulatory bodies.
How long is a GMP certificate valid?
Typically 3 years, with regular surveillance audits in between.
Can small businesses get GMP certified?
Yes. SMEs are increasingly asking for GMP to expand and export.