ISO 9001:2015 Clause 10 – Your Ultimate Guide to Continuous Improvement
Introduction
Striving for excellence with ISO 9001:2015 Clause 10? This guide covers the essentials of continuous improvement, nonconformity, and corrective action. Learn how to identify opportunities, solve problems permanently, and build a quality management system that evolves and enhances customer satisfaction. With Maxicert’s innovative approach to ISO certification and quality system enhancement, businesses are empowered to build dynamic, evolving quality management frameworks that truly elevate customer satisfaction and drive transformative growth.

10 Improvement
Clause 10 of ISO 9001:2015 centers on continuous improvement as a vital part of maintaining and enhancing a quality management system (QMS). It requires organizations to proactively identify improvement opportunities, whether in products, services, or internal processes, to meet customer needs and increase satisfaction. This ongoing improvement effort supports organizational growth and adaptation in a competitive environment.
10.1 General
The organization shall determine and select opportunities for improvement and implement any necessary actions to meet customer requirements and enhance customer satisfaction.
These shall include:
- Improving products and services to meet requirements as well as to address future needs and expectations;
- Correcting, preventing or reducing undesired effects;
- Improving the performance and effectiveness of the quality management system.
NOTE: Examples of improvement can include correction, corrective action, continual improvement, breakthrough change, innovation and re-organization.
What Improvements Can You Make?
Improvement should be interpreted as a recurring activity. What it means is when opportunities for improvement are identified and when such improvements are justified, you need to decide how they are to be implemented, based on the available resources. Where concurrent opportunities are identified, you might need to prioritize their implementation.
It is important to understand that improvement doesn’t necessarily just mean improvement of product, but can also apply to the quality management system itself.
While Corrective action (10.2) identifies methods needed to identifying causes of identified problems (and prevent their recurrence) as is appropriate, improvement is the process of taking actions on a recurring basis to implement agreed solutions that should bring positive benefits.
Steps in the Improvement Process
The process of improvement includes a number of steps, such as:
- Identification of potential opportunities to improve the quality management system,
- Analysis and justification (cost/benefit) to implement an improvement action,
- Determination of the availability of the necessary resources,
- Decision to implement the improvement,
- Implementation of the improvement,
- Measurement of the impact of the improvement,
- Consideration of the results at the next management review.
This clause lists a number of ways that you can use both to plan and actually implement improvement.
ISO 9001:2015 Clause Guide Panel
- Clause 1
- Clause 2
- Clause 3
- Clause 4 – Sub-clause 1
- Clause 4 – Sub-clause 2
- Clause 5 – Sub-clause 1
- Clause 5 – Sub-clause 2
- Clause 5 – Sub-clause 3
- Clause 6 – Sub-clause 1
- Clause 6 – Sub-clause 2
- Clause 7 – Sub-clause 1
- Clause 7 – Sub-clause 2
- Clause 7 – Sub-clause 3
- Clause 7 – Sub-clause 4
- Clause 8 – Sub-clause 1
- Clause 8 – Sub-clause 2
- Clause 8 – Sub-clause 3
- Clause 8 – Sub-clause 4
- Clause 8 – Sub-clause 5
- Clause 8 – Sub-clause 6
- Clause 8 – Sub-clause 7
- Clause 8 – Sub-clause 8
- Clause 8 – Sub-clause 9
- Clause 8 – Sub-clause 10
- Clause 8 – Sub-clause 11
- Clause 8 – Sub-clause 12
- Clause 9 – Sub-clause 1
- Clause 9 – Sub-clause 2
- Clause 9 – Sub-clause 3
- Clause 9 – Sub-clause 4
- Clause 10
Examples of areas where the quality management system can be improved include:
- internal communications,
- follow-up activities,
- documented procedures,
- the effectiveness of management review meetings,
- customer feedback systems, and
- training programs (e.g. for management or for internal auditors).
10.2 Nonconformity and Corrective Action
This section mandates that organizations respond promptly when nonconformities occur by controlling and correcting the issue and addressing the root cause to prevent recurrence. Corrective actions not only fix existing problems but also ensure the quality management system evolves through lessons learned, thereby enhancing overall effectiveness and reducing future risks. Documentation and review of corrective actions are key elements to this process.
Clause Requirements
Corrective action — When a nonconformity occurs
Simple visual steps to react, analyse, act and prevent recurrence (including complaints).
React and control
Take action to control and correct the nonconformity and deal with any consequences (containment, notifications, temporary fixes).
Evaluate need for root-cause action
Review & analyse the nonconformity, determine causes, and check if similar issues exist or could occur elsewhere.
Implement action
Put the required corrective actions into practice (permanent fixes, process changes, training, supplier actions).
Review effectiveness
Check whether the corrective action worked and the nonconformity has been prevented from recurring.
Update risks & opportunities
If needed, update the risks and opportunities identified during planning to reflect the learning from the nonconformity.
Change the QMS
Make necessary changes to the quality management system (procedures, controls, responsibilities) to prevent recurrence.
10.2.2 The organization shall retain documented information as evidence of:
- The nature of the nonconformities and any subsequent actions taken;
- The results of any corrective action.
Correcting the Causes of Problems
The need for corrective action can arise when an internal nonconformity (product or quality management system) occurs, or from external sources such as customer complaints, warranty claims or problems encountered with a supplier.
Corrective action is an important improvement activity. It seeks to eliminate permanently the causes and consequent effects of problems that could have a negative impact on:
- your organization’s results,
- your organization’s products, processes, quality management system, or
- the satisfaction of your customers.
Corrective action involves finding the cause of a particular problem and then putting in place the necessary actions to prevent it from recurring.
Correction vs Corrective Action
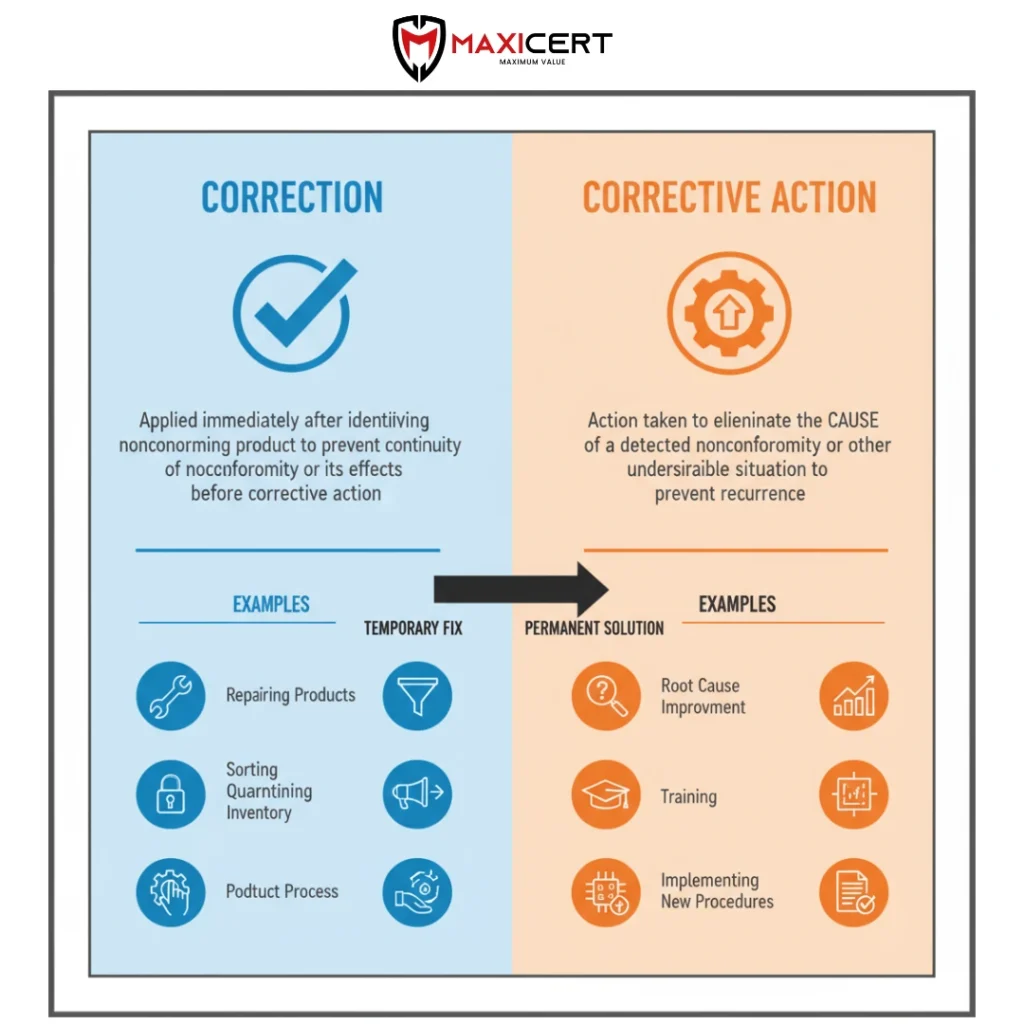
Sources of Corrective Action Needs
The need for corrective action can originate from various sources, such as:
- nonconformities,
- problems with suppliers,
- rework or repairs, or
- customer complaints,
- warranty claims,
- audit reports (see 9.2).
You should record your corrective action activities and follow up the actions to find out whether they have been effective.
Changing Documentation and Resources
You may need to change the quality manual, documented procedures and any other relevant documentation as a result of changes that you have put in place. Changes to such documentation should be made in accordance with the provisions of 7.5.
A documented procedure describing the method you apply for handling corrective actions needs to be established.
It is important that you make adequate resources available in order to ensure the required or agreed corrective action activities can be carried out.
Cost and Risk Considerations in Corrective Action
The size of the problem and the associated risks to your organization will determine the actions that you need to take.
- You do not need to spend large amounts of money on a problem that is costing your organization very little money.
- However, it is important to assess hidden costs before deciding that a problem is not costing too much.
Example: You may be disposing of material as scrap because in its raw state it has negligible value. However, the cost of processing the raw material (equipment time, fossil fuel and labor) may be significant, increasing the value of the scrap and the cost of the problem.
You do need to take care to ensure that the effects of corrective action taken in one area do not cause adverse effects in another area of your organization.
10.3 Continual Improvement
The organization shall continually improve the suitability, adequacy and effectiveness of the quality management system.
The organization shall consider the results of analysis and evaluation, and the outputs from management review, to determine if there are needs or opportunities that shall be addressed as part of continual improvement.
Setting Objectives for Continual Improvement
Continual improvement emanates from the objectives set by top management, which should (at least) address:
- the improvement of internal efficiency (for the organization to remain economically competitive),
- individual customer needs, and
- the level of performance that the market normally expects.
Example: In the aeronautical sector, the acceptable rate of non-conforming delivered product is zero percent. It would not be useful for the organization to set objectives for an “improvement” in this rate. However, it would be useful to have objectives aimed at improving its internal efficiency and competitiveness (e.g. through innovation).
Examples of Areas for Improvement
Examples of areas where the quality management system can be improved include, but are not limited to:
- Internal communications
- Follow-up activities
- Documented procedures
- The effectiveness of management review meetings
- Customer feedback systems and
- Training programs (e.g. for management or for internal auditors).
Continual improvement should be interpreted as a recurring (step by step) activity. What it means is when opportunities for improvement are identified and when such improvements are justified, an organization needs to decide how they are to be implemented, based on the available resources.
Supporting Standards and Guidance
These International Standards can provide assistance to organizations when they are establishing or seeking to improve their quality management systems, their processes or their activities:
- ISO 9000, Quality management systems – Fundamentals and vocabulary
- ISO 9004, Managing for the sustained success of an organization – A quality management approach
- ISO 10001, Quality management – Customer satisfaction – Guidelines for codes of conduct for organizations
- ISO 10002, Quality management – Customer satisfaction – Guidelines for complaints handling in organizations
- ISO 10003, Quality management – Customer satisfaction – Guidelines for dispute resolution external to organizations
- ISO 10004, Quality management – Customer satisfaction – Guidelines for monitoring and measuring
- ISO 10005, Quality management systems – Guidelines for quality plans
- ISO 10006, Quality management systems – Guidelines for quality management in projects
- ISO 10007, Quality management systems – Guidelines for configuration management
- ISO 10008, Quality management – Customer satisfaction – Guidelines for business-to-consumer electronic commerce transactions
- ISO 10012, Measurement management systems – Requirements for measurement processes and measuring equipment
- ISO/TR 10013, Guidelines for quality management system documentation
- ISO 10014, Quality management – Guidelines for realizing financial and economic benefits
- ISO 10015, Quality management – Guidelines for training
- ISO/TR 10017, Guidance on statistical techniques for ISO 9001:2000
- ISO 10018, Quality management – Guidelines on people involvement and competence
- ISO 10019, Guidelines for the selection of quality management system consultants and use of their services
- ISO 14001, Environmental management systems – Requirements with guidance for use
- ISO 19011, Guidelines for auditing management systems
- ISO/DIS 37500, Guidance on outsourcing
- IEC 60300-1, Dependability management – Part 1: Dependability management systems
- IEC 61160, Design review
- ISO/IEC 90003, Software engineering – Guidelines for the application of ISO 9001:2000 to computer software
Conclusion
Mastering Clause 10 is not just ticking boxes—it’s about igniting a culture where every setback is a stepping stone toward innovation. By turning corrective actions into strategic advantages and relentlessly pursuing improvement, your business crafts an agile, resilient quality system that adapts and thrives in a changing world. Partnering with Maxicert means gaining more than certification; it’s about unlocking the potential within your processes, inspiring excellence at every level, and creating a vibrant ecosystem of continuous improvement that secures your organization’s future success.
Free 60–90 day implementation plan available after consultation.
Client Testimonials
What Our Clients Say About Us?
We are trusted by thousands of clients belonging from technology, manufacturing, healthcare and various sectors








Our overall experience with Maxicert was satisfied. The audit and consulting part was handled carefully, we fulfilled our client requirement of ISO 27001 hassle free.
Kevin Santiago BDM – Clarks Outsourcing, PhilippinesTimely response and knowledge of ISO standards can be seen together in the team of Maxicert, we grow because of the service providers like Maxicert.
Samuel Christopher Quality Assurance Head – OEQA, NigeriaWe did Food safety certification with Maxicert, the service was extraordinary and their consultant had good experience of the subject.
Mr. Venkatesh Production Manager - Acacia Foods and Beverages, ZambiaWe engaged a consultant of Maxicert for our business certification, we now have a well-designed and organized department procedures and we rectify our errors through internal audits regularly.
Abdullah Al Rayes Managing Director – TCS, BahrainTechnical expertise by the team of Maxicert helped us achieving our ISO 13485 certificates, we now proudly say that we have achieved our target, all thanks to the team.
Nady Boustany CEO – LMG, IraqMaxiCert's approach to meet our needs proved instrumental in facilitating a seamless transition throughout the entire ISO certification process for us. Their training sessions are so much helpful.
Ms. Latifa Al Salem Investor portfolio – Ministry of Investment, Saudi ArabiaMaxicert is a one stop solution, we got trainings, documents, audit and certification at one place, they facilitated everything.
Ms. Mariam Chaggama VP – Fasthub, TanzaniaFAQ
What is the main goal of Clause 10 in ISO 9001?
Clause 10 focuses on continuously improving your quality management system, products, and services to better meet customer needs and enhance satisfaction.
What should an organization do when a problem or nonconformity occurs?
The organization must take corrective action by finding the root cause, fixing the problem, and making sure it doesn’t happen again.
How is continual improvement different from just fixing problems?
Continual improvement means regularly looking for new ways to do better, not just reacting to issues. It’s about making your processes, products, and system better step by step over time.
Why is employee involvement important in continual improvement?
Employees can spot improvement opportunities and provide ideas that help the whole organization grow. Their participation creates a culture of quality and innovation.





Their presence in Oman made us even better to accomplish our goal of achieving ISO certificates on time, we will definitely recommend their services.
Mr. Sailesh Mohanakrishnan Division Manager – Khimji Ramdas, Oman