ISO 14971 Certification: A Complete Guide to Risk Management for Medical Devices

Introduction
When people’s lives are on the line, there is no chance for failure. From plain syringes to sophisticated heart monitors, all medical devices need to adhere to the highest safety standards. So how do companies guarantee that their devices are safe for their lifecycle? The solution is ISO 14971, the globally accepted standard for risk management of medical devices.
Here, we’ll guide you through what ISO 14971 is, why it is important, its principal requirements, and how it can assist your company. Whether you’re new to the medical device industry or want to enhance compliance, this guide will offer useful information.
What is ISO 14971?
ISO 14971 is the international standard for medical device risk management. It offers a framework that assists manufacturers in identifying potential hazards, estimating and assessing risks, applying control measures, and monitoring the effectiveness of the controls.
The initial ISO 14971 was published in 2000, and since then, the standard has advanced to keep pace with emergent technologies and regulation demands. Now, it is used extensively by manufacturers in need of market approval in regions such as the European Union, the United States, and several other countries.
ISO 14971 is not only about complying with regulatory requirements—it’s about designing safer devices that command the respect of healthcare professionals and patients.
Request A Free Quote
Why Risk Management is Critical in Medical Devices
The medical device market is distinctive in that the risk of failure or malfunction has the potential to be so catastrophic as to include injury or death. A defect or omission in a medical device can have a direct impact on patient safety and health, unlike most products.
Take the case of a defective implant or an ailing infusion pump. These can result in product recalls, litigation, and damage to reputation. Most importantly, they jeopardize patients’ health.
That is why risk management, as understood in ISO 14971, is at the center of medical device development. It makes sure that risks are detected early and reduced before they have the ability to lead to harm. Indeed, risk management is so significant that it is a main requirement under regulatory systems like the EU Medical Device Regulation (MDR) and the US FDA’s quality system demands. View guidance from the European Medicines Agency.
Key Requirements of ISO 14971
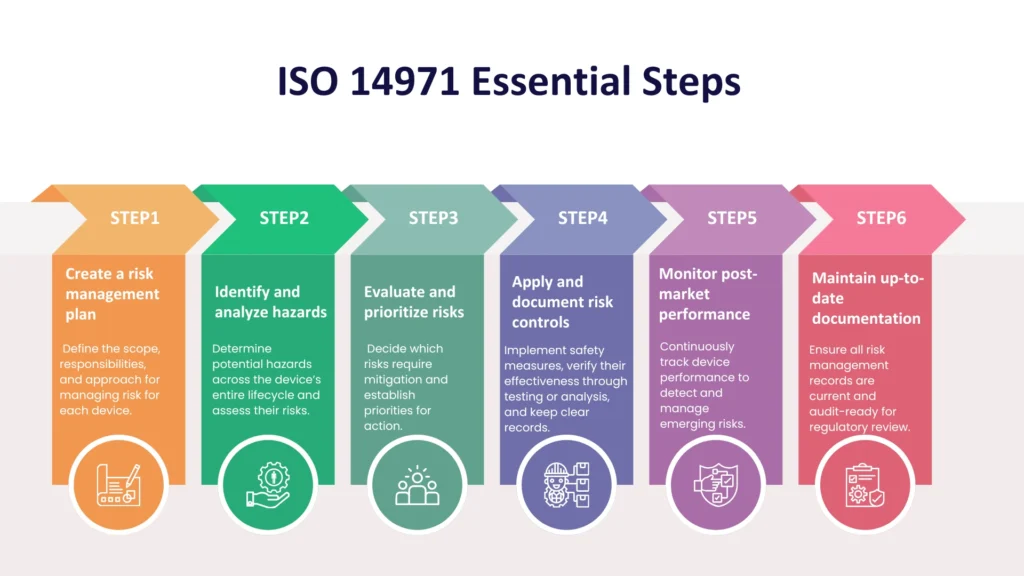
The ISO 14971 standard provides a step-by-step method of managing risks and applies to the entire life cycle of a medical device. The following are what it entails:
- Risk analysis: This is where possible hazards to the device, like mechanical failure, software malfunction, or biological hazards, are identified. Manufacturers then have to quantify the risk from each hazard using the severity of harm and likelihood of occurrence.
- Risk assessment: After risks have been identified, manufacturers need to decide which risks are acceptable and which need more control. Acceptability criteria are typically established in the risk management plan.
- Risk reduction: For unacceptable risks, manufacturers need to introduce measures to lower them. This might involve design modifications, protective elements, or warning signs.
- Effectiveness of risk controls: Placing controls is not sufficient. The effectiveness of the controls should be validated by means of testing or analysis.
- Risk assessment of residual risk: Manufacturers are required to evaluate any residual risk once the controls have been implemented and determine whether it is tolerable.
- Manufacturing and post-manufacturing activities: ISO 14971 focuses on ongoing monitoring. Manufacturers are required to gather and analyze production data as well as real-world usage of the device in order to determine new or previously unknown risks.
This organized process prevents any risk from being missed and ensures that safety is given utmost consideration from design development up to the retirement of the device.
ISO 14971 Compliance Process
It takes commitment, planning, and constant effort to implement ISO 14971. Here is a simplified step-by-step plan:
ISO 14971 vs. Other Standards
It’s useful to see how ISO 14971 sits within the broader landscape of medical device standards. Whereas ISO 13485 targets quality management systems, ISO 14971 targets risk. They both complement each other to deliver an overarching system for ensuring device quality and safety.
Find out more about ISO 13485 certification.
ISO 14971 also supplements regulatory guidelines like the EU MDR, which requires risk management across the product’s lifecycle.
Advantages of ISO 14971 Certification
Implementing ISO 14971 encompasses a broad array of advantages:
- Improved patient safety: Systematic risk management results in safer devices and reduced incidents.
- Compliance with regulations: Adhering to ISO 14971 can simplify approval procedures in major markets.
- Customer confidence: Certification conveys to customers and business partners that your products are created with safety as the top priority
- Advantage over competitors: Organizations with effective risk management tend to secure more business, particularly in highly regulated environments.
How Maxicert Can Assist with ISO 14971 Certification
ISO 14971 requirements may seem daunting, particularly for businesses that are just starting in the medical device industry or venturing into new markets. That’s where Maxicert is here to help.
We offer assistance, including:
- Establishing a compliant risk management system on your behalf
- Documenting risk files and supporting documentation
- Educating your employees about ISO 14971 processes and best practices
- Accompanying you through audits and regulatory examinations
Discover our ISO Certification Services to find out how we can aid your compliance process.
Conclusion
Risk management is no longer an option in today’s medical device market. ISO 14971 offers a clear way to safer products, easier regulatory clearances, and enhanced market credibility.
Let Maxicert assist you in moving forward. Contact us today for professional guidance on your ISO 14971 compliance process. Together, we can create safer, more reliable medical devices.

Get In Touch

Get In Touch

Get In Touch
Need A Free Estimate?
Get a free consultation and Checklist to get certified for ISO , HALAL, CE Mark Certification.
FAQ
Is ISO 14971 a requirement for every medical device manufacturer?
Yes, ISO 14971 is technically voluntary, but it is cited in numerous regulatory guidelines as the standard approach for risk management. Compliance with it is usually necessary to gain market approval.
How much time does it take to implement ISO 14971?
Implementation time depends on how complex your device is and how complex your current processes are. Small manufacturers might take a couple of months, and larger companies might take longer to embed risk management entirely.
Can small medical device companies take advantage of ISO 14971?
Absolutely! ISO 14971 is adaptable. Small companies can implement its principles in a manner that is appropriate for their size and means but that also fulfills regulatory requirements.
Is ISO 14971 only for finished medical device manufacturers?
No. ISO 14971 is for everyone involved in the lifecycle of the device, including designers, suppliers, and providers of services, where risk management is essential to ensure device safety.