GMP MANUFACTURING Certification in Philippines: Overview & Benefits

Introduction
In the health, pharmaceutical, and food industries, where competition is sharp and regulations are strict, maintaining quality and safety often determines a brand’s success. This is particularly true in the Philippines, where consumer awareness, government policies, and trade standards are tightening.
This is where GMP Certification shines. It is not simply a certificate; it stands as a mark of trust, hygiene, quality, and international standards. For food processors, drug manufacturers, or cosmetic brands, acquiring a GMP certification is a strategic approach to expand business while ensuring customer protection.
What is GMP Manufacturing?
To define, GMP (Good Manufacturing Practices) are industry-specific norms that include standards and procedures aimed at ensuring uniform production and control of goods. These standards span the entire production sequence — raw materials, packaging, and shipping.
Key Elements of GMP:
- Clean workplace to avoid contamination.
- Sufficiently maintained equipment.
- Skilled workers who have received the appropriate training.
- Controlled processes of production and validated methods.
- Precise record keeping and identifiability at every step.
GMP provides the framework for systematic processes in order to create safe and quality products.
See detailed information about GMP Manufacturing on Wikipedia.
Request A Free Quote
The Importance of GMP Certification in the Philippines
In the Philippines, the focus of GMP certification arises from:
- The surge in needs for a safe and high-quality food and drug products.
- The growing public demand for safe and trusted brands and reliable products.
- Increased export-driven companies seeking access to global markets.
- Increased regulatory scrutiny from the Philippine FDA and DOH (Department of Health).
- Government oversight caused by food safety scandals and pharmaceutical recall crises.
- GMP certification has been shown to safeguard a business’s license during inspections.
- In most local and foreign markets, it serves as a prerequisite for registration of the product.
GMP Certification Process in the Philippines
Every process starts with specific steps that will make your organization meet the requirements of local and international GMP standards.
1. Gap Analysis
— A detailed examination of your manufacturing processes focusing on the gaps or inefficiencies.
2. Documentation
— Arrange the following supporting documents to be collected:
- SOPs (Standard Operating Procedures)
- Quality Assurance Manual
- Manufacturing batch records
- Risk assessments and product traceability files
3. Staff Training
— Train all relevant personnel on aspects such as:
- Good hygiene practices
- Correct usage of equipment
- SOP adherence and corrective action protocols
4. Implementation
— Revise the facility’s layout for the e and packaging flow, and incorporate procurement, testing, and distribution to meet GMP standards.
5. Internal & External Audit
— Perform a mock audit first and then invite a certified external auditing body to complete the official audit to the inspection.
6. Continual Improvement
— Post certification, ensure to frequently evaluate your procedures in order to remain compliant.
Industries That Require GMP Certification
Public safety has a direct correlation with manufacturing industries which use GMP certification as a quality aid.
- Pharmaceuticals: Tablet and injectable manufacturing, R&D labs.
- Nutraceuticals & Dietary Supplements: Capsule, syrup, and powder production.
- Food & Beverages: Packaged foods, frozen goods, dairy, beverages.
- Cosmetics & Personal Care: Skin creams, soaps, shampoos, oral care.
- Traditional Medicine & Ayurveda: Herbal products, essential oils, tonics.
If your product is ingested, applied to skin, or injected — GMP is vital.
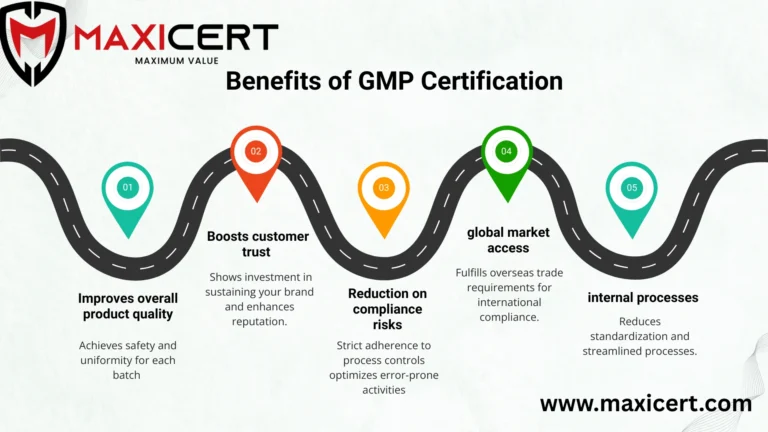
Benefits of GMP Certification
Common Myths About GMP Certification
- GMP is only for large businesses — Even small businesses benefit from it.
- GMP is not a perpetual process — It’s an ongoing commitment to quality.
- GMP is solely documentation focused — It also covers hygiene, facility design, equipment, and personnel.
- GMP is an expensive endeavor — It saves money long-term by reducing risks and waste.
- A certification will provide a company with no defects — It significantly reduces risk but does not eliminate it entirely.
How to Choose the Right Certification Partner
Selecting a trustworthy certification partner can either propel or hinder your progress in GMP. Consider the following:
- Accreditation: Ensure that the partner is recognized by local and international institutions such as the FDA and ISO.
- Industry Experience:Inquire about their experience in your field.
- Training Support: Find providers that offer additional services like training, gap analysis, and mock audits.
- Transparency & Support:Make sure they clearly communicate the timelines, costs, and any assistance available after certification.
Maxicert is your reliable GMP Certification partner in the Philippines.
Tips for a Successful GMP Audit
Prepare for the audit day stress. Follow these strategies to keep yourself perpetually prepared:
- Maintain up-to-date SOPs and logs.
- Perform internal audits or mock inspections every three months.
- Educate personnel on emergency and hygiene protocol compliance.
- Storage and labeling accuracy, along with equipment maintenance, should always be optimal.
- Identify blind spots using GMP readiness checklists.
Remember: Consistency is the key to a successful audit!
Conclusion
Having GMP Manufacturing Certification strengthens product assurance, safety, and relentless enhancement. It is no longer an optional regulatory requirement in the Philippines’s competitive markets of food, pharmaceuticals, and cosmetics. More businesses are beginning to understand the value of being GMP compliant.
Looking for a partner to get your business GMP certified in the Philippines? From training to audit, Maxicert helps you every step of the way as a trusted certification service provider.
Reach out to us now and get a free consultation to help you make your brand compliant for global expansion!

Get In Touch

Get In Touch

Get In Touch
Need A Free Estimate?
Get a free consultation and Checklist to get certified for ISO , HALAL, CE Mark Certification.
FAQ
What is GMP Certification?
GMP Certification is a document issued to a manufacturer as a result of an audit as a verification that the proper procedures are put in place for manufacturing processes which ensures safety, quality and uniformity in the products.
Is GMP Certification mandatory in the Philippines?
For sectors such as food, drugs and cosmetics, yes, it is mandatory. Moreover, it is often a prerequisite for FDA product clearance in a number of situations.
How long does it take to get GMP certified in the Philippines?
It is usually between 3 to 6 months, depending on the readiness and intricacy of the company.
What are the costs involved in GMP Certification?
These costs differ based on the size of the company, the condition of the facility, the training required attending, and documentation efforts. Maxicert has reasonable packages designed for small and medium enterprises.